Enzyme QMS Software
Enzyme's QMS is built by industry veterans frustrated by existing QMS solutions.
We designed it for all life science companies, paying particular attention to the challenges building FDA-regulated software.
Out-of-the-Box Solution
Enzyme’s QMS includes modules that cover the key components of 21 CFR 820 and ISO 13485
Design Control
CAPAs
Complaints
Document Control

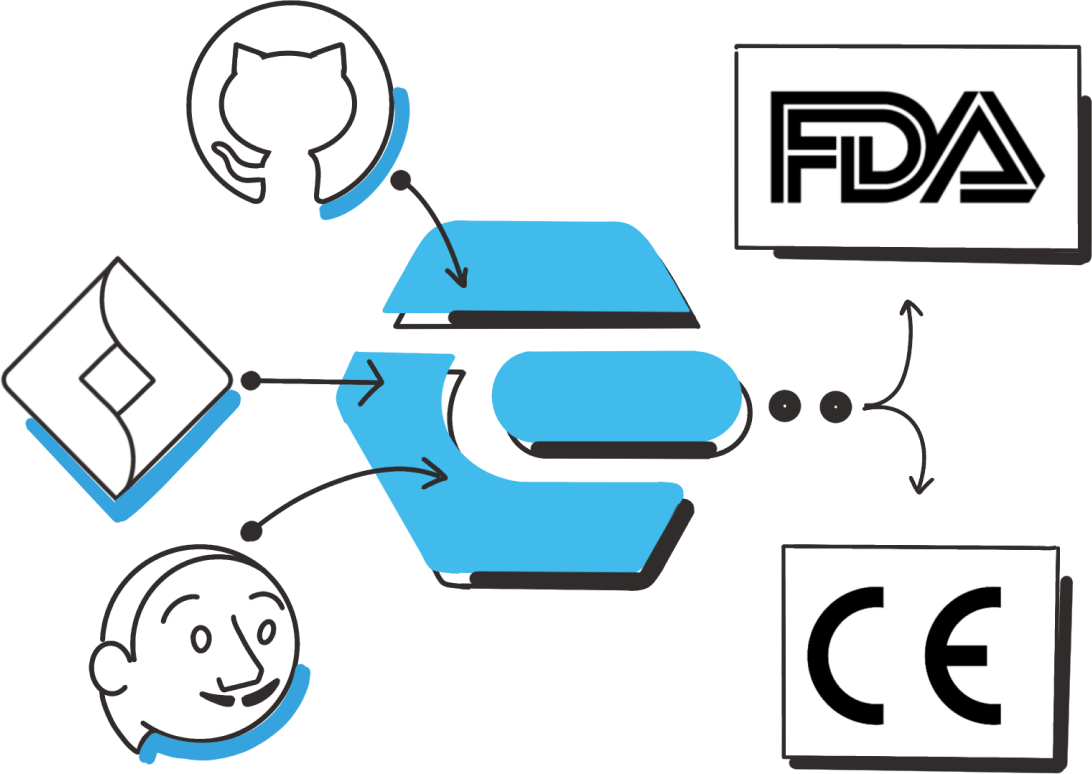
Integrates with your existing tools
- Our QMS is designed for use in a modern, agile software development process
- Imports existing data into Enzyme so you don’t have to repeat the work
- Generate reports directly from Enzyme which can be used for submission

Quality System Template Library
- Enzyme offers a complete set of SOPs, Work Instructions, Quality Manuals and forms
- Enzyme Consulting, LLC. can support you in customization and implementation your Quality System
- The templates support innovative products - whether its traditional, SaMD or hybrid of the two
Onboard your team swiftly
- Enzyme offers a comprehensive onboarding process
- We train you on the modules you need first
- After your onboarding, Enzyme’s team is available for follow up calls and to answer your questions